Feline Infectious Peritonitis Virus RTPCR Detection
Introduction
Feline infectious peritonitis virus (FIPV) is a pathogen that causes feline infectious peritonitis (FIP) and septicemia in cats. The virus is widespread globally, and there is currently no specific treatment for it. RT-PCR is a highly sensitive, specific, and effective detection method that has been widely used for detecting FIPV nucleic acids. The detection method primarily includes steps such as sample collection and processing, RNA extraction, RTPCR reaction system preparation, and detection. Among these, RNA extraction is one of the most critical steps in the detection process and is usually performed using commercially available RNA extraction kits. This detection method offers numerous advantages, including efficiency, speed, accuracy, and ease of operation. However, there are some limitations, such as requiring professional laboratories and equipment, high technical skill, and the inability to distinguish between live and dead viruses. RT-PCR is a reliable diagnostic method for FIPV, enabling the early detection and control of disease spread.

1. Overview of Feline Infectious Peritonitis Virus
Feline infectious peritonitis is common in young and elderly cats. The virus is widespread globally and poses a threat to household cats. FIPV belongs to the coronavirus family and is highly contagious. It spreads through saliva, nasal discharge, and tears, and can survive for a long time in contaminated environments, making cats easily susceptible to cross-infection. After cats are infected with FIPV, the initial symptoms resemble those of a common cold, but the condition gradually worsens, leading to fever, anemia, weight loss, and abdominal fluid accumulation. Treatment for FIPV is extremely difficult, and there is currently no specific medication to cure the disease. Treatment mainly focuses on relieving symptoms, but most infected cats eventually die. To prevent and control feline infectious peritonitis, maintaining environmental hygiene, personal hygiene for cats, and vaccination are important. Additionally, if a cat exhibits related symptoms, it is essential to seek help from a veterinary clinic. Once diagnosed, infected cats should be isolated to prevent further spread of the disease【1】.
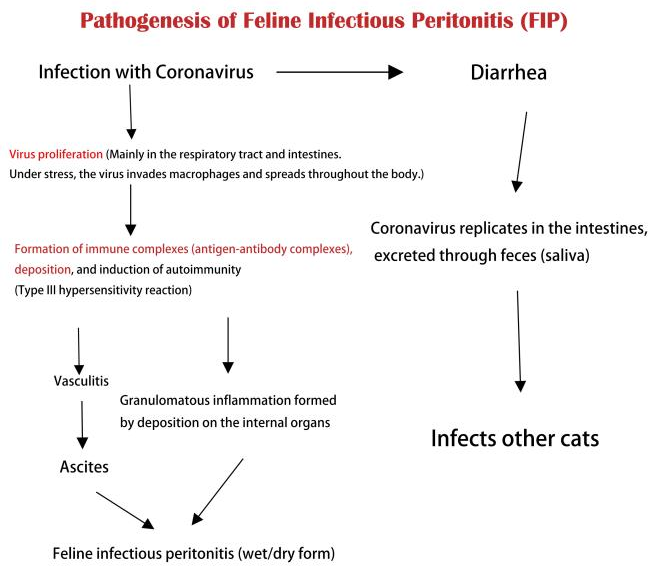
2. Principle and Characteristics of RTPCR
Reverse Transcription Polymerase Chain Reaction (RTPCR) is a variant of PCR technology, a method that uses reverse transcriptase to convert RNA into cDNA, and then amplifies specific DNA sequences through polymerase chain reaction. Its principle is essentially the same as PCR technology, but differs in the sample processing step.
RTPCR mainly involves three steps: RNA reverse transcription, cDNA amplification, and sequencing and identification. First, reverse transcriptase and primers are added to the sample, and through reverse transcription, RNA is transcribed into cDNA. Then, during the PCR process, primers are used to replicate the cDNA into a large number of DNA fragments. Finally, by comparing the amplified products from the sample with those from a positive control, it can be determined whether the target DNA or RNA sequence is present.
RTPCR technology has the following advantages: it can detect low-abundance RNA or DNA, rapidly and efficiently amplifies target sequences, and requires only a very small amount of starting DNA template without needing to purify and amplify large amounts of template DNA. It can also detect multiple targets simultaneously, yielding multiple results in a single experiment. The results are stable and reliable, making it suitable for various fields of modern medicine and life sciences.
Although RTPCR technology is highly powerful, it requires precise laboratory operations, specialized equipment, primer design, and hybridization suppression mechanisms. Additionally, it requires evaluation of the reaction system and the establishment of strict operating procedures, such as QSOP or SOP. In summary, as a highly sensitive and specific detection method, RTPCR has been widely applied and promoted in numerous fields【2】.
Table 1: RTPCR Principles and Characteristics
| Characteristic | RTPCR Principle |
|---|---|
| Sensitivity | Highly sensitive, capable of detecting very low concentrations of viral nucleic acids. |
| Specificity | Strong specificity, able to distinguish between different types of viruses. |
| Accuracy | Highly accurate, with reliable results and minimal error. |
| Operational Complexity | Relatively complex to operate, requiring highly specialized laboratories and technical expertise. |
| Time Cost | Relatively high, ranging from several hours to up to two days. |
| Reliability | Stable results, not easily affected by external factors. |
| Application Range | Widely used in the detection of viral nucleic acids, including COVID-19, influenza, and others. |
The above table outlines the primary characteristics and principles of RTPCR technology. Overall, RTPCR is one of the most widely used methods for viral nucleic acid detection, known for its sensitivity, accuracy, and reliability, though it requires specialized equipment and personnel to perform.
3. RTPCR Detection Method for Feline Infectious Peritonitis Virus (FIPV)
3.1 Sample Collection and Processing
The process for sample collection and processing in FIPV RTPCR detection is as follows:
Sample Selection: The test samples should be collected from cats showing symptoms of feline infectious peritonitis (FIP) or those that have lived with an infected cat.
Sample Collection: Samples can be obtained from oral, nasal, or rectal secretions, or from tissue. Sterile swabs should be used, and areas with a high presence of normal bacteria should be avoided.
Sample Processing: After collection, samples must be sent immediately to the laboratory for processing. For biological tissues, RNA extraction kits or other methods should be used to isolate the RNA. For body fluids, direct treatment using RNAse and DNAse-free buffers can capture transcripts efficiently.
Reverse Transcription Reaction: The extracted RNA is reverse transcribed into cDNA, typically using random or specific primers. The reverse transcription reaction can be performed with a PCR machine and generally follows these parameters:
- 25-42°C for 10-60 minutes (RNase H)
- 50-98°C for 10-15 minutes (Denaturation)
- 5°C to stop the reaction
PCR Amplification: It’s recommended to perform early detection on cDNA using probes such as SYBR Green or other detection methods to analyze specific PCR products.
3.2 RNA Extraction
The RNA extraction procedure in FIPV RTPCR detection consists of the following steps:
Sample Collection: Samples such as ascites, pleural fluid, exudate, tissue, or blood are collected from cats showing symptoms of FIP or suspected to be infected with FIPV. Care must be taken to avoid contamination and interference from foreign substances.
RNA Extraction: Total RNA is extracted from the collected samples using appropriate RNA extraction kits or other methods. The process must strictly control bacterial, RNase, and external DNA/RNA contamination to ensure the purity and integrity of the pathogen’s RNA.
Storage: The extracted RNA can either be used immediately for reverse transcription PCR analysis or stored for future use【3】. For RNA stability and integrity, storage should be at -80°C or in liquid nitrogen. The specific steps and details of RNA extraction may vary depending on the sample type and the RNA extraction kit used, so it is essential to read the relevant kit instructions carefully or seek technical assistance. To ensure the accuracy of the test results, nucleic acid analysis equipment and reagents should be used in a designated nucleic acid work area, following standard operating procedures and protocols.
3.3 RTPCR Reaction System Preparation
Preparation of PCR Reagents: According to the chosen PCR method and kit instructions, prepare the PCR reaction mixture. Typically, the PCR reaction mixture consists of template DNA/RNA, primers, enzymes, buffers, and dNTPs.
Preparation of Reverse Transcription System: Prepare the reverse transcription reaction mixture. The reverse transcription reaction generally includes an RNA template, reverse transcriptase, and primers. To prevent contamination by exogenous nucleic acids, RNase inhibitors can be used to avoid RNA degradation during the reverse transcription process.
Mixing PCR and Reverse Transcription Reaction Mixtures: Combine the prepared PCR reaction mixture and reverse transcription reaction mixture.
Addition of Samples as Required: Add the extracted RNA from the samples into the tube containing the PCR and reverse transcription reaction mixtures. The sample volume may need to be adjusted according to different sample types.
Performing the PCR Cycles: The program involves cycles of denaturation, annealing, and extension. These three steps are repeated continuously, with enzyme activity controlled by the temperature in the thermal cycler.
Post-PCR Analysis: After completing the PCR cycles, the PCR products can be detected via gel electrophoresis or other methods.
In summary, during the preparation of the RTPCR reaction system, it is essential to control the purity of materials, adhere to strict operating procedures, and maintain lab hygiene to ensure the reliability and accuracy of the test results.
Table 2: RTPCR Detection Methods for Feline Infectious Peritonitis Virus (FIPV)
| Detection Method | Advantages | Disadvantages |
|---|---|---|
| Real-time Fluorescent PCR | High sensitivity, good specificity | High sample requirements, easily affected by contamination, complex operation |
| Gradient PCR | Simple operation, low cost | Sensitivity is lower than real-time fluorescent PCR |
| Conventional PCR | Simple operation, low cost | Low sensitivity, specificity is not as good as real-time fluorescent PCR |
| Magnetic Bead Quick Separation RTPCR | Convenient operation, fast detection time | High sample requirements, easily affected by contamination |
| Endonuclease Restriction Site Analysis | Simple detection, both sensitivity and specificity are high | Results require electrophoresis analysis, complex operation |
| Block PCR | Can analyze amplified fragments, useful for determining virus strain types and subtypes | Complex operation, amplification primer design is complicated |
4. Result Analysis
4.1 Common Possible Outcomes
Negative: No FIPV RNA was detected, indicating no occurrence of FIP.
False Negative: Improper sample handling, low viral load, or technical issues could lead to a negative result, even though the cat carries FIPV.
Positive: FIPV RNA is detected in the sample. In this case, the result should be considered along with the cat’s condition and other test results to make a final diagnosis of feline infectious peritonitis (FIP)【4】.
4.2 Points to Note
A positive test result does not definitively confirm the presence of FIP, as the cat may be in the early stages of FIPV infection without showing clinical signs. For a positive result, it is essential to combine other diagnostic information, such as clinical symptoms, hematological tests, and pathological examinations, to assess whether FIP is truly present. The analysis of FIPV RTPCR test results should always be performed alongside clinical symptoms and additional diagnostic findings to make an informed judgment about whether the cat is infected with FIP.
5. Application of RT-PCR in the Diagnosis of Feline Infectious Peritonitis
5.1 Advantages
High Sensitivity: Compared to other detection methods, RTPCR technology is quicker and more accurate in detecting target viral nucleic acids.
Strong Specificity: Since the PCR reaction only amplifies specific fragments of DNA or RNA, RTPCR is widely used for its specificity.
Simple Operation: Compared to other diagnostic methods in the lab, RTPCR requires fewer materials and is relatively straightforward to perform.
Rapid Results: Unlike other molecular biology techniques, RTPCR provides reliable results in a short period【5】.
5.2 Disadvantages
High Sample Requirement: RTPCR requires appropriate samples from the infected cat, such as ascites, pleural fluid, effusion, and tissues. Collecting such samples can be challenging and requires professional handling to avoid false-negative results.
Special Equipment Needs: The PCR procedure requires high-quality equipment and a controlled environment. Preventing contamination is crucial, and the operator must be experienced and follow a strict protocol.
Inability to Differentiate Active Infection: Detecting FIPV RNA alone cannot distinguish other gastrointestinal and diarrheal diseases caused by non-infectious factors. Therefore, it must be used in conjunction with other diagnostic methods.
Conclusion
RTPCR is an efficient, fast, accurate, and reliable detection method that holds significant clinical importance in diagnosing feline infectious peritonitis (FIP). However, it is crucial to note that RTPCR test results should be combined with other relevant examinations and clinical manifestations to develop a comprehensive diagnosis and treatment plan. When analyzing RTPCR results for Feline Infectious Peritonitis Virus (FIPV), the findings must be evaluated alongside the cat’s clinical presentation and additional diagnostic results to make a final determination on whether the cat is infected with FIP.
References
[1]Yang Ni, Han Diangang, Yang Yunqing, et al. Establishment of Quantitative Fluorescence RT-PCR Detection Method for Feline Infectious Peritonitis Virus [J]. China Animal Quarantine, 2022, 39(10):127-131.
[2]Wang Yuanhong. Isolation and Identification of the Feline Infectious Peritonitis Virus HF1902 Strain and Establishment of SYBR Green Detection Method [D]. Anhui Agricultural University, 2020. DOI:10.26919/d.cnki.gannu.2020.000178.
[3]Li Jian, Chen Qin, Wang Quan, et al. Establishment of RT-PCR Detection Method for Feline Coronavirus and Feline Infectious Peritonitis Virus [J]. Animal Husbandry and Veterinary Medicine, 2019(11):32-34.
[4]Lü Yanli, Chen Xiaoyun, Guo Xin, et al. RT-PCR Detection of Feline Infectious Peritonitis Virus [C]//Chinese Association of Animal Husbandry and Veterinary Medicine, Sichuan Agricultural University. Proceedings of the 6th National Congress of the Chinese Association of Animal Infectious Diseases and the 11th Academic Seminar. [Publisher not specified], 2005:3.
[5]Ishida T, Hong Lei. Detection of Feline Infectious Peritonitis Virus Antibody by Enzyme-Linked Immunosorbent Assay (ELISA) [J]. Veterinary Drug Bulletin, 2022, No.82(02):52-54.

