Effectiveness Analysis of Portable Real-Time Fluorescent PCR Instrument for Detecting Animal Viruses
Introduction
Animal viruses pose a significant threat to animal health and livestock production. Their spread and prevalence in the farming and livestock industries have a profound impact on human productivity, livelihoods, and public health. To ensure the safety of animal health and livestock production, the detection of animal viruses has become an essential task. Traditional methods for animal virus detection often require lengthy experimental processes, extended data processing times, and high technical expertise, making them inadequate for the rapid identification of animal viruses. In response, researchers have increasingly turned to more efficient detection methods, leading to the development of the portable real-time fluorescent PCR instrument[1].
The portable real-time fluorescent PCR instrument is an efficient, rapid, and accurate tool for detecting animal viruses. It minimizes the risks of cross-contamination during the testing process and delivers reliable results within a short time frame. Additionally, it is not restricted by location, enabling on-site testing in environments such as transport vehicles or field settings.
1. Basic Principles of Real-time Fluorescent PCR Technology
Real-time fluorescent PCR is a rapid, sensitive, and specific method for detecting target DNA or RNA sequences. It combines PCR amplification with real-time fluorescent probe technology to monitor the fluorescent signal generated during the thermal cycling of the PCR reaction. This allows for the real-time quantification of the target template concentration during the PCR process.
The main components of a real-time fluorescent PCR reaction include the template DNA or RNA, primers, probes, and fluorescent dyes. Primers are necessary for the PCR amplification process, as they pair with the start and end points of the target sequence to generate the desired amplification products. Probes are highly labeled molecules that detect the specific binding events of the target sequence during PCR amplification, indicating the progress of the reaction. Fluorescent dyes are molecules that strongly absorb light, transferring the fluorescence signal from the primers and probes to specific detectors through a process called fluorescence resonance energy transfer (FRET), thus enabling detection[2].
In a real-time fluorescent PCR reaction, the intensity of the fluorescent signal is measured to determine the presence and concentration of the amplification products. At the end of each PCR cycle, the system collects and quantifies the generated fluorescent signals, converting them into digital outputs (i.e., fluorescence quantification). Using real-time fluorescence signals and quantification, the gene count of the target sequence is calculated. Figure 1 below shows the schematic of a portable real-time fluorescent PCR instrument.
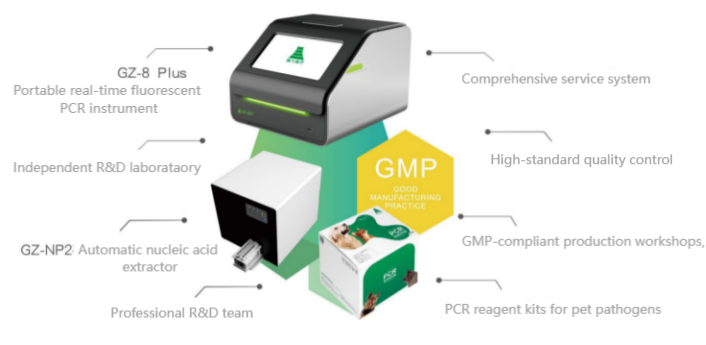
2. Research Methods
To evaluate the effectiveness of the portable real-time fluorescent PCR instrument in detecting animal viruses, this study employed methods such as sample collection and processing, the design of real-time fluorescent PCR detection protocols, and the use of specific equipment and reagents. The detailed steps of each method are described below, followed by data analysis of the experimental results.
2.1 Sample Collection and Processing
This study selected three typical animal viruses—Avibacterium paragallinarum, bovine viral diarrhea virus (BVDV), and classical swine fever virus (CSFV)—as the subjects for investigation. The corresponding standard samples were collected for detection as follows:
Avibacterium paragallinarum standard: Samples were taken from easily infected tissues such as blood and organs, processed, and genetic material extracted for detection.
BVDV standard: Samples were taken from the intestines and other tissues of deceased cattle, processed, and genetic material extracted for detection.
CSFV standard: Samples were collected from whole-body tissues of deceased pigs, processed, and genetic material extracted for detection.
2.2 Real-time Fluorescent PCR Detection Protocol Design
The study designed three detection protocols corresponding to the target viruses: Avibacterium paragallinarum, bovine viral diarrhea virus, and classical swine fever virus. The detection protocols are as follows:
Avibacterium paragallinarum Detection Protocol:
- Preprocessing: Sample subjected to cell lysis.
- Real-time Fluorescent PCR: PCR amplification using specific primers and probes, with real-time fluorescence detection using SYBR Green.
BVDV Detection Protocol:
- Preprocessing: Sample subjected to nucleic acid extraction.
- Real-time Fluorescent PCR: PCR amplification using specific primers and probes, with real-time fluorescence detection using SYBR Green.
CSFV Detection Protocol:
- Preprocessing: Sample subjected to nucleic acid extraction.
- Real-time Fluorescent PCR: PCR amplification using specific primers and probes, with real-time fluorescence detection using FAM[3].
2.3 Equipment and Reagent Usage Instructions
Real-time Fluorescent PCR Instrument: The portable real-time fluorescent PCR instrument was used for detection. In this experiment, the MA-1600Q series portable real-time fluorescent PCR instrument, developed by Yaray Bio based on the classic MA-6000 series, was utilized. The MA-1600Q series can be equipped with up to three fluorescent detection channels and comes with a standard 16-well detection throughput. All experimental operations and result analyses can be completed via the built-in touchscreen. The compact design and optional external battery make it highly adaptable to user needs. Some technical specifications of the MA-1620Q/1630Q portable real-time fluorescent PCR instrument are shown in Table 1.
Table 1. Partial Technical Specifications of the MA-1620Q/1630Q Portable Real-time Fluorescent PCR Instrument
| Instrument Model | MA-1620Q | MA-1630Q |
|---|---|---|
| Sample Capacity | 16×0.2ml, compatible with single tubes and 8-strip tubes | |
| Heating Method | Peltier heating method with unique edge heat protection and intelligent temperature control technology ensuring rapid and uniform heating and cooling, providing uniform well-to-well temperatures and eliminating edge effects. | |
| Temperature Control Range | 4℃~99℃ | |
| Heating Rate | 3.2℃/S(MAX) | |
For the detection of Avibacterium paragallinarum standard samples, commercially available PCR kits such as the GeneAmp PCR System 9700 or SYBR PCR Master Mix (Applied Biosystems) can be used. For nucleic acid extraction, the QIAamp DNA Blood Mini Kit (Qiagen) is recommended, as it recovers high-purity DNA from Avibacterium paragallinarum-containing blood samples efficiently, while minimizing repetitive procedures and preserving DNA quality.
For detecting bovine viral diarrhea virus (BVDV) standard samples, commercially available Real-time PCR kits such as Maxima SYBR Green/ROX qPCR Master Mix can be utilized, which are suitable for detecting low concentrations of BVDV RNA. Nucleic acid extraction can be performed using commercially available kits like the QIAamp Viral RNA Mini Kit, which purifies RNA from tissues such as the intestines, ideal for BVDV detection.
For the detection of classical swine fever virus (CSFV) standard samples, Real-time PCR kits such as SYBR Green-based kits, like GoTaq Probe qPCR Master Mix (Promega), can be employed. Nucleic acid extraction can be performed using the QIAamp DNA/RNA Mini Kit (Qiagen), which extracts both DNA and RNA from samples of deceased pigs, enabling successful CSFV detection.
SYBR Green: Used for real-time fluorescence quantification.
FAM: Used for real-time fluorescence detection of CSFV.
3. Experimental Results
3.1 Experimental Findings
In this study, a portable real-time fluorescent PCR instrument was used to detect standard samples of animal viruses, including Avibacterium paragallinarum, bovine viral diarrhea virus (BVDV), and classical swine fever virus (CSFV). The results showed successful detection of all the standard samples, with the detection limits of the real-time fluorescent PCR technology being lower than those of traditional PCR methods. The minimum detection limits were as follows:
Avibacterium paragallinarum: 10 CFU/ml
BVDV: 100 fg/ml
CSFV: 50 fg/ml
A comparison between the detection limits of real-time fluorescent PCR and traditional PCR revealed that the former is more sensitive and accurate, as well as faster and simpler to operate. In detecting Avibacterium paragallinarum, BVDV, and CSFV, the detection limits for traditional PCR were 100 CFU/ml, 500 fg/ml, and 1000 fg/ml, respectively. Therefore, real-time fluorescent PCR technology proves to be superior for animal virus detection and holds great potential for widespread applications.
Additionally, the portable real-time fluorescent PCR instrument is compact and easy to use, making it ideal for mobile laboratories and on-site testing. Whether for clinical diagnostics or monitoring in the livestock industry, real-time fluorescent PCR provides essential support for animal disease prevention and control[4]. In conclusion, real-time fluorescent PCR is an effective method for detecting animal viruses and has promising future applications.
3.2 Advantages of the Portable Real-time Fluorescent PCR Instrument
The portable real-time fluorescent PCR method has extensive applications in virus monitoring and clinical diagnostics. Compared to traditional virus detection methods, real-time fluorescent PCR offers the following advantages:
High Sensitivity: Real-time fluorescent PCR can detect very low concentrations of viruses, typically at CFU/ml or even fg/ml levels, facilitating rapid detection of viral cases.
High Specificity: The specially designed primers used in real-time fluorescent PCR precisely target specific viral sequences for amplification, greatly reducing the likelihood of false positives.
Real-time Monitoring: Real-time fluorescent PCR allows the accumulation of amplification products to be monitored in real time, enabling immediate results after the reaction, significantly increasing detection efficiency.
Precise Quantification: Real-time fluorescent PCR quantifies the target virus concentration based on the intensity of the fluorescent signal, providing accurate measurements.
High Reliability: The technique demonstrates high reproducibility and accuracy in multiple tests, ensuring consistent and reliable results.
4.Improvement and Optimization Suggestions for the Portable Real-time Fluorescent PCR Instrument
The portable real-time fluorescent PCR instrument holds vast potential in animal virus detection, but continuous improvements and optimizations are necessary. The following are suggestions for enhancement:
4.1 Increase Detection Speed:
Although the current portable real-time fluorescent PCR instruments offer rapid detection, further optimization can be achieved. By refining the PCR reaction system, adjusting the PCR cycling program, and optimizing the scanning speed, the virus standard samples and actual samples could be detected even faster.
4.2 Enhance Detection Sensitivity:
While the instrument already delivers high sensitivity, there are scenarios where even greater sensitivity is required. Improvements to the PCR reaction system, optimization in the selection of primers and probes, and refinement of experimental protocols can further enhance sensitivity to meet the demands of more stringent detection needs[5].
4.3 Reduce Detection Costs:
Developing more cost-effective detection protocols and simplifying the use of PCR reagents and systems can help reduce the overall cost of detection without compromising accuracy. Additionally, creating specialized PCR reagents and primers for specific tests could meet particular detection needs while keeping costs low.
In conclusion, while the portable real-time fluorescent PCR instrument is widely applicable in animal virus detection, ongoing optimization is necessary to enhance detection efficiency, lower costs, and increase detection speed. This will enable the instrument to play a more significant role in animal disease prevention and control.
Conclusion
In this study, a portable real-time fluorescent PCR instrument was used to detect three animal viruses. The experimental results demonstrated that the instrument can rapidly and accurately detect target viruses with high sensitivity and specificity. Compared to traditional PCR methods, real-time fluorescent PCR offers higher sensitivity and accuracy, making it more suitable for clinical diagnostics and monitoring in animal disease prevention.
Additionally, the portable real-time fluorescent PCR instrument is user-friendly, highly mobile, and well-suited for on-site testing and mobile laboratories, providing better support for animal disease prevention efforts. Therefore, it can be concluded that the portable real-time fluorescent PCR instrument is an efficient, convenient, rapid, and accurate tool for detecting animal viruses, and it is highly applicable for the diagnosis and monitoring of animal diseases.
References
[1] Yang Aifu, Sun Yun, Wan Chao, Liu Xuehua, Tai Limei, Wang Mei. Establishment and Application of TaqMan Probe Real-time Fluorescent PCR Detection Method for Black Truffle Components [J/OL]. Journal of Chinese Food Science: 1-10 [2023-06-09].
[2] Zhu Yuli, An Run, Feng Zhihui. Identification of Di(b-) Rare Blood Type by TaqMan-MGB Real-time Fluorescent PCR Method [J]. Journal of Clinical Blood Transfusion and Laboratory Medicine, 2023, 25(02): 235-238.
[3] Liu Libing, Zhou Cang, Fu Qi, Sun Xiaoxia, Wang Jinfeng, Qian Yunkai, Ai Lianfeng, Wang Jianchang. Establishment and Preliminary Application of Real-time Fluorescent PCR Technology for Detecting Four Common High-histamine Fish Components [J]. Chinese Port Science and Technology, 2023, 5(03): 50-58.
[4] Zhang Yuexin. Discussion on the Effectiveness of Real-time Quantitative PCR Instrument for Influenza Virus Detection [J]. China Medical Device Information, 2022, 28(02): 62-64.
[5] Yun Kaiyi, Xiang Li, Wang Xiaoyue, Liu Yang, Yao Hui, Song Jingyuan. Identification Study of Cordyceps Sinensis and Its Adulterants Based on Portable and CFX96 Real-time Fluorescent PCR Instruments [J]. Acta Pharmaceutica Sinica, 2019, 54(04): 746-752.